What Do We Know?
As you have learned, when the concentration of atmospheric carbon dioxide increases, the concentration of carbonate ions in the ocean decreases. This reduces the availability of the carbonate ions necessary for marine animals to form their shells.
However, the decreased concentrations of carbonate also affect the dissolution equilibrium of calcium carbonate.
Your Turn
Calcium carbonate reaches equilibrium with aquated calcium and carbonate ions in ocean water, as shown below.
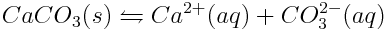
Because the concentration of carbon dioxide in the atmosphere is increasing, the concentration of carbonate ions in the ocean is decreasing. As the concentration of carbonate ions decreases, how will the dissolution equilibrium of calcium carbonate shift? How will this affect the shells of marine animals?
As the concentration of carbonate ions decreases, the concentration of reactants is lowered and the reaction quotient, Q, of the dissolution reaction decreases. To approach equilibrium, solid calcium carbonate must dissolve, increasing the concentrations of products so that Q increases to approach Ksp. The shells of marine animals are made primarily of calcium carbonate and may begin to dissolve as the water in their ocean habitat attempts to restore equilibrium.