Why Should We Care?
Human activity is disturbing the reaction between gaseous carbon dioxide and aqueous carbon dioxide, by increasing the concentration of carbon dioxide in the atmosphere, primarily through the combustion of fossil fuels.
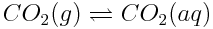

By increasing the concentration of the reactant, CO2(g), humans are decreasing the value of Q. Therefore, the reaction must shift to increase Q, so that Q=K. To increase Q, more products must be formed and more carbon dioxide dissolves into the ocean.
Worked Example
Human activity adds carbon dioxide to the atmosphere, which results in an increase in the amount of carbon dioxide dissolved in the ocean. Recall that after carbon dioxide dissolves in the ocean it proceeds to take part in the following series of related reactions:
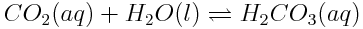
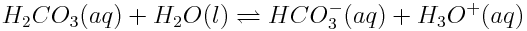
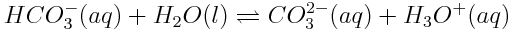
How will the increasing concentration of dissolved carbon dioxide shift the entire series of equilibria as shown above? How will the pH of the ocean be affected?
The increased concentration of dissolved carbon dioxide will shift each of the above reactions to the products.
- The increased concentration of CO2(aq) reduces Q for the first reaction. To increase Q\Q, more products must be formed, so the concentration of H2CO3(aq) increases.
- The increased concentration of H2CO3(aq) reduces Q for the second reaction. To increase Q, more products must be formed, so the concentrations of HCO3-(aq) and H3O+(aq) increase.
- The increased concentration of HCO3-(aq) reduces Q for the third reaction. However, the increased concentration of H3O+(aq) increases Q for the reaction. Thus it is difficult to predict which direction this equilibrium will go, because both the numerator and denominator increase. In reality, there are other reactions, which occur after this step, which cause this reaction quotient to decrease, so the forward reaction becomes favoured.
In the final two reactions in the series, hydronium ions are produced. Because the increased concentration of dissolved carbon dioxide shifts these reactions to the product side, the number of hydronium ions that is produced continually increases. A greater concentration of hydronium ions translates into a lower ocean pH.
Note: Although this reaction series indicates that the concentration of carbonate ions (CO32-) increases due to increased concentrations of dissolved carbon dioxide in the ocean, other reactions in the ocean actually cause the concentration of carbonate ions to decrease as carbon dioxide enters the ocean. The reactions above are important in decreasing ocean pH, but other reactions play a more important role in determining the concentrations of carbonate ions. This subject will be discussed in Key Idea 6.
Question For Thought
How do you think the acidification of the ocean affects marine animals?