The process through which increasing atmospheric carbon dioxide concentrations lowers the concentration of carbonate ions is fairly complicated. This is because many reactions occur between the various carbonate species as well as between carbonate species and other species in ocean water. However, for illustrative purposes, let us explore the following reaction that is important in regulating the concentration of carbonate ions:
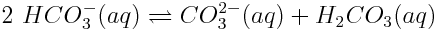
How will this reaction respond to an increase in atmospheric carbon dioxide concentration?
Recall that, as the concentration of gaseous carbon dioxide in the atmosphere increases, the concentration of dissolved carbon dioxide in the ocean also increases. Also, recall that dissolved carbon dioxide reacts with water to produce carbonic acid:
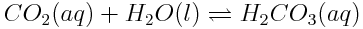
Therefore, as the concentration of aqueous carbon dioxide increases, the value of Q changes, and the reaction shifts to increase the concentration of carbonic acid.
When the concentration of carbonic acid increases, the Q value of the first reaction increases because more products are present. The reaction will shift to produce more reactants. However, as this occurs, carbonate ions react, and the concentration of these ions decreases.
Although this is a simplification of the process by which carbon speciation is affected by atmospheric carbon dioxide, it illustrates the overall result: As the atmospheric concentration of carbon dioxide increases, the concentration of carbonate ions in the ocean decreases.