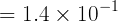Worked Example
The speciation of carbon in the oceans has important effects on marine life. Below is a speciation plot showing how pH affects the distribution of HA(aq), a weak acid, and A-(aq), the conjugate base.

- Estimate the pKa and Ka of the weak acid.
- At what pH is [HA]/[A-]=100/1?
- At what pH does [HA]/[A-]=1/1000?
- Remember that, when [HA]=[A-], pH=pKa. For this speciation plot, the pH when the two curves intersect is about 7.4. Therefore, pKa is 7.4 and Ka = 10-7.4> = 4.0×10-8.
-
Recall that the equation
describes pH-dependent speciation of a weak acid and its conjugate base. From this equation, we can determine pH when [HA]/[A-]=100/1, using the Ka determined in the question above:

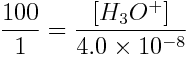
When [H3O+]=4.0×10-6 mol L-1,
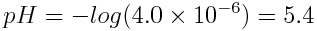
-


When [H3O+]=4.0×10-11 mol L-1,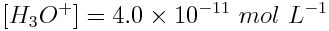

Your Turn
Although carbonate species are changing ocean pH, boric acid (H3BO3) and aquated borate ions (B(OH)4-) also play a key role in determining ocean pH. The Ka of boric acid is 7.24×10-10. Based on this information, determine the H3BO3/ B(OH)4- ratio at the following pH values:
- pH 4.00
- pH 7.00
- pH 10.00
-
At pH 4, [H3O+] = 10-4 = 1.0×10-4 mol L-1.
The H3BO3/B(OH)4- ratio is,


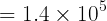
-
At pH 7, [H3O+] = 10-7 = 1.0×10-7 mol L-1.
The H3BO3/B(OH)4- ratio is,



-
At pH 10, [H3O+] = 10-10 = 1.0×10-10 mol L-1.
The H3BO3/B(OH)4- ratio is,