What Do We Know?
One of the forms or polymorphs of calcium carbonate used by marine animals is called aragonite. Aragonite is a solid, but it is slightly soluble in water; low concentrations of its ions can be found in solution. Aragonite’s dissolution reaction is shown below:
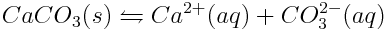
The equilibrium constant of this reaction tells us the extent to which aragonite dissolves in water. In this case the equilibrium constant is called a solubility product (Ksp) because it describes the solubility of a compound in water. The solubility product of aragonite, in surface seawater at typical temperatures and salinity, is:
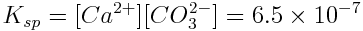
Note that the solubility product is very small. The concentration of products that are formed is small, and aragonite is not very soluble in water. Also, note that calcium carbonate is not included in the calculation of the solubility product because it is a solid.
The solubility of aragonite and its solubility product (Ksp) are determined through experimental measurements of the concentrations of calcium and carbonate ions in a saturated solution. When a solution is saturated with aragonite, the above reaction is at equilibrium and Q=Ksp. Then, no more CaCO3 should dissolve.
However, in the water at the ocean’s surface the dissolution of aragonite is not at equilibrium.