What Do We Know?
The observation that many properties of gases are independent of the identity of the gas has allowed chemists to develop the 'ideal' gas model. This model helps us conceptually and mathematically understand the bulk properties of gases. An ideal gas is defined as a gas in which the molecules of the gas are extremely small compared to the volume of the container and the molecules do not experience intermolecular forces. Although no gas is ever completely ideal, this model is still useful in predicting and understanding the bulk properties of gases. It can often be used to explain the behavior of real gases quite well under certain conditions of pressure and temperature.
Making the assumption that gases are ideal has allowed chemists to establish the relationship between the bulk properties of gases. These relationships are combined into the ideal gas law, shown below:
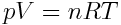
In the ideal gas law equation, p is pressure, V is volume, n is the amount of the gas in moles, R is the universal gas constant (8.314 L kPa K-1 mol-1) and T is temperature in Kelvin.