As the water molecules in a boiler absorb the energy released by burning coal, the molecules gain kinetic energy and move faster and faster. This increase in kinetic energy causes an increase in the water’s thermal energy as well as the water’s temperature. However, thermal energy and temperature must be carefully differentiated. While water’s thermal energy refers to the total kinetic energy of the water molecules in the sample, temperature refers to the average kinetic energy of the water molecules. Therefore, a boiler containing 150 L of water at 100 °C holds more thermal energy than a boiler of water containing 75 L at the same temperature. The average kinetic energies of the particles in each boiler are the same, so that they have the same temperature, but the larger boiler holds more water particles and, therefore, more thermal energy.
By absorbing energy in the boiler, the water vaporizes and is passed over the blades of a turbine inside the generator
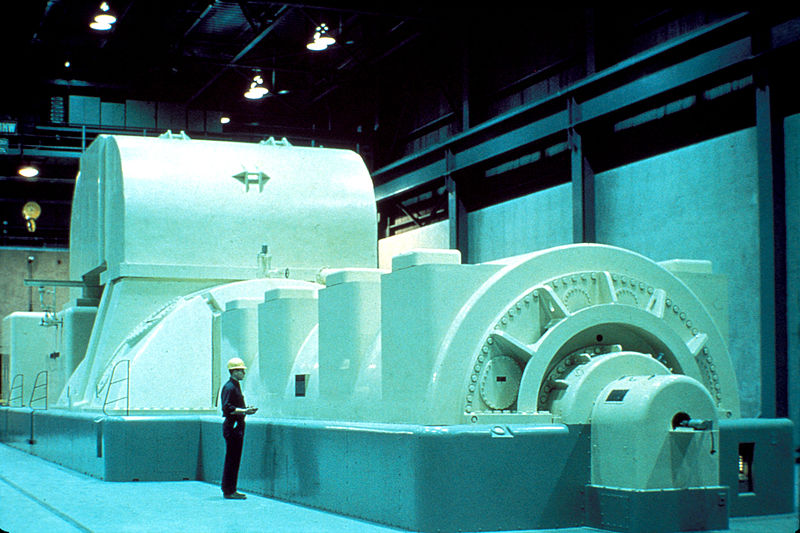 (a modern steam turbine generator is shown to the right). As a result, the kinetic energy of the water molecules is converted into the kinetic energy of the turbine, causing the turbine to spin. As the turbine turns the wire coil inside a generator, the kinetic energy of the turbine fins is transformed into electrical energy, the energy associated with the flow of electrons. This electrical energy is transported along electrical transmission lines until, inside your house, the heater converts electrical energy into thermal energy-increased motion of the atoms in the heater wire. The increased thermal energy in the wire is transferred to the air inside the house, causing the kinetic energy of the air molecules and, consequently, the thermal energy of the air to increase.
(a modern steam turbine generator is shown to the right). As a result, the kinetic energy of the water molecules is converted into the kinetic energy of the turbine, causing the turbine to spin. As the turbine turns the wire coil inside a generator, the kinetic energy of the turbine fins is transformed into electrical energy, the energy associated with the flow of electrons. This electrical energy is transported along electrical transmission lines until, inside your house, the heater converts electrical energy into thermal energy-increased motion of the atoms in the heater wire. The increased thermal energy in the wire is transferred to the air inside the house, causing the kinetic energy of the air molecules and, consequently, the thermal energy of the air to increase.