During a phase change, heat is absorbed or released because the intermolecular forces within the substance must be strengthened or weakened to produce the phase change. Because the transfer of energy during a phase change affects intermolecular forces rather than the actual movement of the particles, and in a system where the phases are allowed to exchange energy with the rest of the system, the potential energies of the molecules change, while the average kinetic energies stay the same. Since the average kinetic energy of the molecules in a substance stays the same, the temperature of a substance is constant during a phase change.
How do we know?
The fact that temperature remains constant while a substance undergoes a phase change can be strikingly portrayed with an IR imaging camera, which you were introduced to in Key Idea 2. Recall that, in an IR image, the temperature of an object is indicated by its color. Look at the following images of ice placed within a crystallization dish and set on a hot plate.
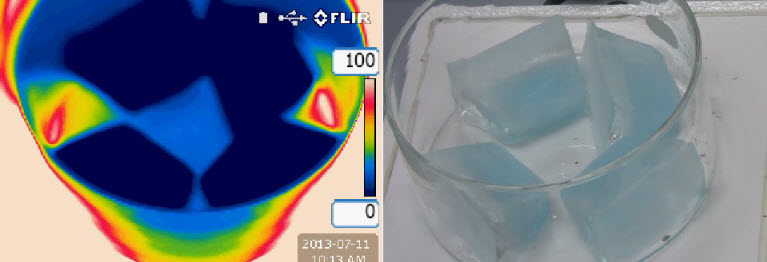
Based on the IR image above, how can you tell that the ice is colder than the crystallization dish?
According to the temperature scale on the right of the IR image, the coldest objects in the image are very dark blue. While the ice is dark blue, no part of the dish is dark blue; therefore, the ice must be colder than the dish.
What do the light colored, whitish regions on the IR image indicate? Hint: Compare the positions of the light colored areas to the visible light image.
The light colored regions indicate areas of very high temperatures surrounding the crystallization dish. Because they surround the crystallization dish, these whitish regions correspond to the hot plate. We can conclude that the hot plate is turned on and hot.