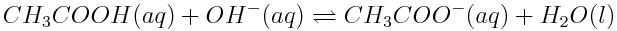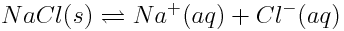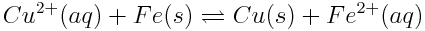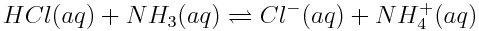What Do We Know?
One model for understanding acid-base reactions defines them as reactions that transfer protons (H+ ions). Acids and bases compete for protons. In a reaction, the species that donates a proton is called the acid and the species that accepts a proton is identified as the base.
Acid-base reactions are defined as proton transfer reactions according to the Bronsted-Lowry model of acids and bases. Chemists have developed other models of acids and bases, in order to explain their behavior at the molecular level. However, we will focus our discussion of acids and bases on the Brønsted-Lowry model. Experimental data has shown that this model is valid under many circumstances and it provides a fairly simple explanation for the behavior of many acids and bases important to our understanding of ocean chemistry.
Worked Example
The reaction between carbonic acid and water shown below is one of several important reactions that control the acidity of the ocean. According to the Brønsted-Lowry model, this is an acid-base reaction because H+ ions are transferred between the reactants.
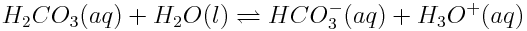
Identify the acid and base in the reaction of the above reaction. Justify your choices.
Your Turn
Changing concentrations of atmospheric carbon dioxide are affecting the balance of acid-base reactions in the ocean, with the net effect of lowering the pH of the oceans. This increase in acidity has detrimental effects on marine life. Which of the reactions shown below are acid-base reactions? How do you know?