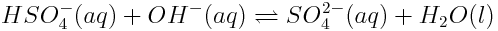What Do We Know?
All acid-base reactions share an important feature: conjugate acid-base pairs. According to the Bronsted-Lowry model, a conjugate acid-base pair refers to two species that differ by the gain or loss of a single H+ ion. Take a look at the reaction involving hydrogen carbonate written below. As you already know, this reaction is involved in determining the pH of the oceans.
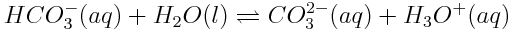
In this reaction, HCO3- and CO32- differ by a single proton. Therefore, we can identify them as a conjugate acid-base pair; HCO3- is the acid and CO32- is the conjugate base. H2O and H3O+ are also a conjugate acid-base pair; H2O acts as the base, while H3O+ is the conjugate acid.
Your Turn
Increased carbon dioxide levels in the atmosphere have increased the concentration of acidic species in the ocean. In each of the following equations, identify the acid on the left side and its conjugate base on the right. Also, identify the base on the left and its conjugate acid on the right.
- HCOOH is the acid that donates a proton to form its conjugate base HCOO-; H2O acts as a base, accepting a proton to form its conjugate acid H3O+.
- H2S is the acid that donates a proton to form its conjugate base HS-; NH3 is the base, accepting a proton to form its conjugate acid NH4+.
- HSO4- is the acid that donates a proton to form its conjugate base SO42-; OH- is the base, accepting a proton to form its conjugate acid H2O.