What Do We Know?
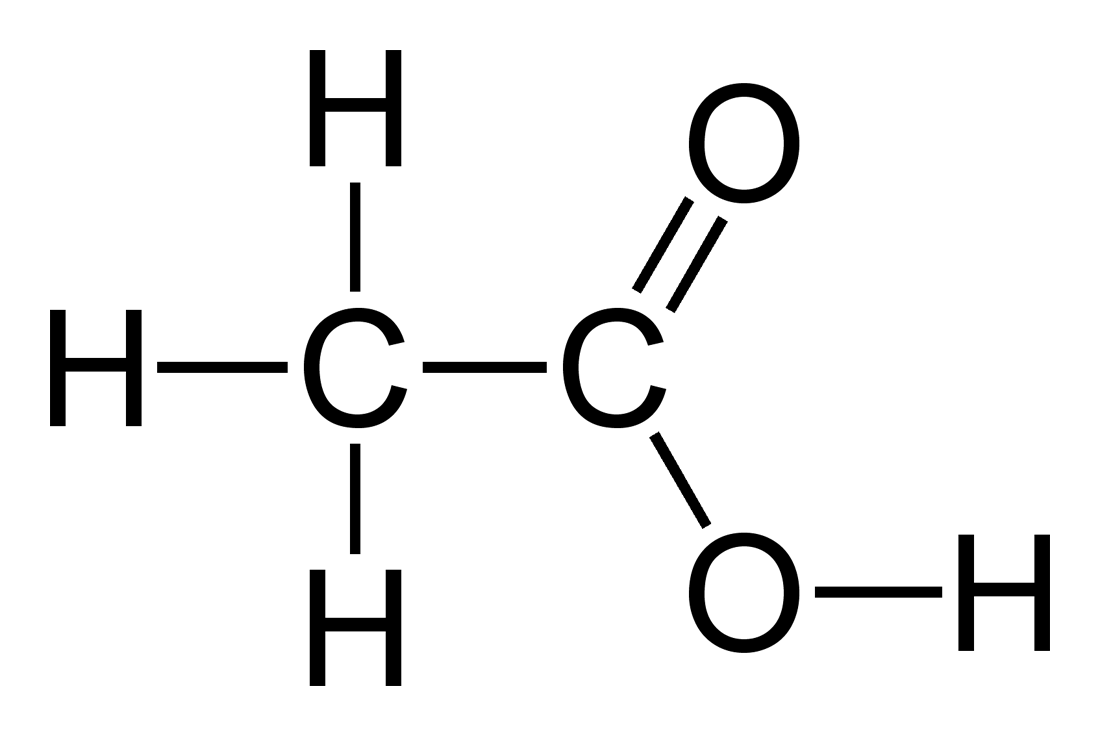
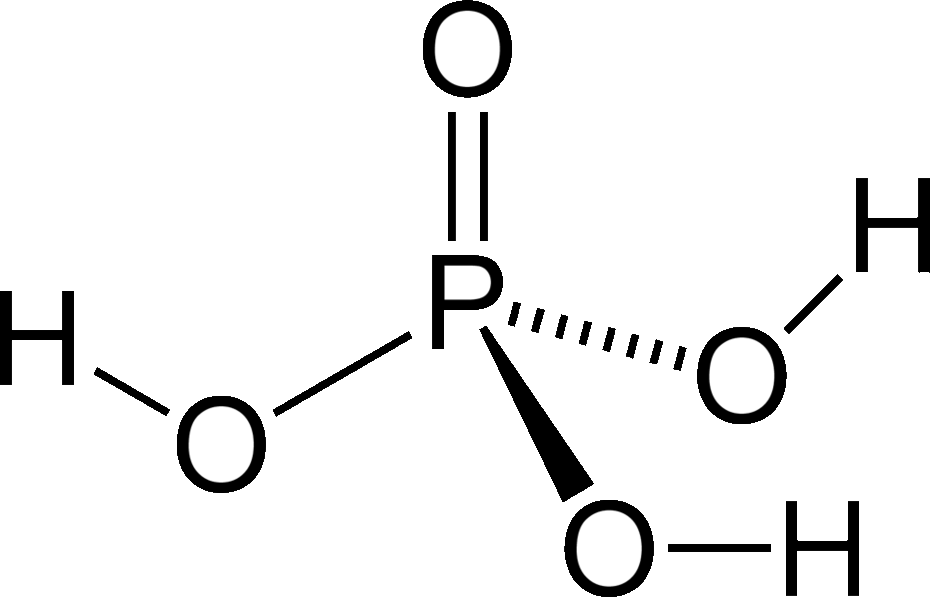
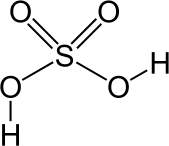
Some acids contain more than one acidic proton that can be donated in acid-base reactions. Monoprotic acids only have one H+ ion to donate, while polyprotic acids can donate more than one H+ ion. For example, hydrochloric acid (HCl) is a monoprotic acid because each molecule of this acid can only donate one hydrogen ion. By contrast, carbonic acid (H2CO3), which plays an important role in the acidification of the ocean, is a polyprotic acid (specifically a diprotic acid) because each molecule of this acid can donate two hydrogen ions.
Your Turn
An understanding of acids has enabled chemists to discern how atmospheric carbon dioxide is lowering the pH of the ocean. Of the following acids, which are monoprotic and which are polyprotic?

Some compounds are amphoteric. Amphoteric species can act as acids or bases because they can either accept or donate a proton, depending on the surrounding environment. For example, water (H2O) is amphoteric. You have seen water act as a base when it forms hydronium ions (H3O+) in the reactions involved in determining the pH of the ocean. However, water can also act as an acid by donating a proton to form hydroxide ions (OH-).
Another important amphoteric species is hydrogen carbonate (HCO3-). As noted previously, hydrogen carbonate often acts as an acid in the ocean, being deprotonated by water to create carbonate and hydronium ions. However, in the presence of strong acid, hydrogen carbonate can act as a proton acceptor, to create carbonic acid.
Your Turn
Hydrogen carbonate ions (HCO3-) play an important role in the acidification of the ocean. Write two balanced equations demonstrating the ability of HCO3- to act as an acid or a base. What is the name for species, like HCO3-, that can act as both an acid and a base?
Hydrogen carbonate acts as an acid in the following reaction:
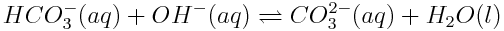
Hydrogen carbonate acts as a base in the following reaction:
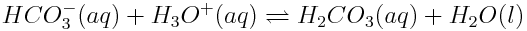
Species that can act as either an acid or a base are called amphoteric species.