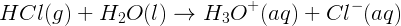What Do We Know?
The concentrations of reactants and products at equilibrium is not the same for all reactions. Some reactions “go to completion”. This means that, at equilibrium, the reaction mixture consists of virtually all products and no reactants. For example, the reaction between hydrogen chloride, a strong acid, and water essentially goes to completion:
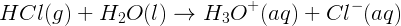
In other reactions at equilibrium, hardly any products have been formed and essentially only reactants are present. For example, at room temperature and pressure, the reaction between nitrogen gas and hydrogen gas to produce ammonia for fertilizer does not produce significant amounts of product:
Most reactions lie somewhere between the two examples shown above. At equilibrium, both reactants and products are present to some degree. However, whether the concentration of products is greater than the concentration of reactants, or vice versa, depends on the specific reaction that is occurring and the surrounding conditions. For instance, as you saw previously, both gaseous carbon dioxide and dissolved or aqueous carbon dioxide are present when the reaction shown below is at equilibrium.