What Do We Know?
To quantitatively describe chemical reactions, chemists have developed a function called the reaction quotient, Q. The reaction quotient compares the ratio of reactant and product concentrations as a reaction proceeds. To explore the significance of the reaction quotient, we will analyze the reaction between hydrogen gas (H2) and iodine gas (I2) at 425 °C.
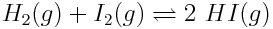
For this reaction, the reaction quotient is:
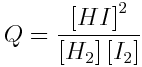
Worked Example
The reaction quotient is an important tool that chemists use to understand equilibria, including the acid-base equilibria that contribute to the acidification of the ocean. Suppose 0.0175 mol L-1 of hydrogen gas and 0.0175 mol L-1 of iodine gas are mixed in a container. Assume the reaction is,
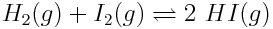
What is the value of the reaction quotient, Q, at the very beginning of the reaction, before any hydrogen iodide gas has been produced?
Simply substitute the concentrations of H2(g), I2(g) and HI(g) into the reaction quotient expression. No hydrogen iodide gas (HI) has been produced, so [HI]=0.