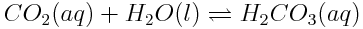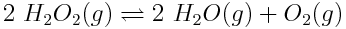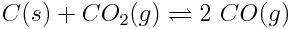The expressions for reaction quotients and equilibrium constants can be applied to any reaction. In general, for the reaction,

The reaction quotient is:
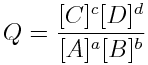
And, at equilibrium, the reaction quotient becomes the equilibrium constant, so,

Two important rules apply to writing the reaction quotients for reactions:
- Solids are not included in the reaction quotient expression because the concentrations of solids do not change during a reaction.
- For reactions occurring in aqueous solution, liquid water is usually not included in the reaction quotient expression because the concentration of liquid water effectively does not change even when it is involved in the reaction.
Why are units not included in the reaction quotient? The simple answer is that keeping track of units at this point will introduce serious problems to more advanced calculations. More accurate calculations of reaction quotients which account for the concentration units ultimately result in unit-less reaction quotients. In this resource, we assume that the units of the reaction quotients and equilibrium constants can be ignored.
Worked Example
A basic understanding of equilibria and reaction quotients is important to understanding acids and the acidity of the ocean. Write the reaction quotient expression for each of the following reactions.

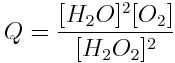
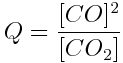
Note: Remember that liquid water and solids are not included in the reaction quotient expression, because their concentrations do not change during a reaction.
Your Turn
Consider the following reaction:
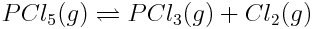
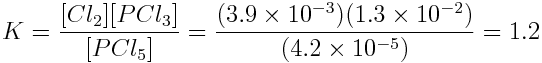
Content subject to KCVS terms of use.
Click here to see our land acknowledgement.
© The King's Centre for Visualization in Science.