Suppose another reaction mixture is formed with unequal concentrations of hydrogen and iodine gas, consisting of [H2]=0.0200 mol L-1and [I2]=0.0100 mol L-1. Initially, no hydrogen iodide gas is formed, so Q=0. As before, as the Q increases as the reaction proceeds. At equilibrium, measurements indicate that [H2]=0.0106 mol L-1, [I2]=0.00060 mol L-1 and [HI]=0.0188 mol L-1. We can then calculate the reaction quotient at equilibrium:
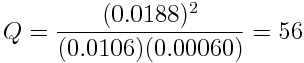
At equilibrium, the value of the reaction quotient, Q, is the same even though different starting reaction mixtures were used. In fact, for this reaction, the value of Q at equilibrium will always be 56. It doesn't matter what concentrations of reactants or products are present in the initial reaction mixture.
This example illustrates the law of equilibrium: For a specified reaction at a certain temperature, the reaction quotient (Q) will be the same value for all reaction mixtures when equilibrium has been reached. At this point, the reaction quotient is called the equilibrium constant, K.
Experimental measurements of the concentrations of reactants and products prove that, at equilibrium for a given reaction at a certain temperature, the reaction quotient consistently reaches a constant value. This value is the same,regardless of the initial concentrations of reactants and products.