What Do We Know?
The reason that acids and bases have such different uses primarily resides in the varying strengths of acids and bases.
In aqueous solutions, the strength of an acid depends on the extent of the reaction between the acid and water.
Strong acids ionize completely in solution through an acid-base reaction with water and, therefore, are strong electrolytes. Reactions between strong acids and water “go to completion”; therefore, the equilibrium constant (K) is large. An example of a strong acid is hydrogen chloride, which is called hydrochloric acid when in solution. Hydrogen chloride undergoes the following reaction:
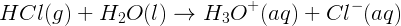
On the other hand, weak acids are not strong electrolytes and only partially ionize in solution when reacting with water in an acid-base reaction. Carbonic acid, which is involved in determining the pH of the ocean, is a weak acid and does not ionize completely in solution in the reaction shown below:
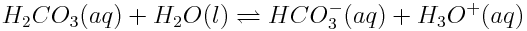
Because this reaction does not go to completion, a significant concentration of carbonic acid remains in solution, which then breaks down into water and carbon dioxide. In reactions between weak acids and water, K is less than 1 because most of the acid is not ionized.