What Do We Know?
The strength of strong acids is essentially the same because they all ionize almost completely in water. However, weak acids do not react completely with water. At equilibrium in solutions of weak acids, both the acid and its conjugate base are present. How can the extent to which these acids and bases ionize in water be compared? One way to compare the relative strengths of weak acids is to react the same volume of water with various acids of equal concentrations and then measure the resulting pH. The weak acid that produces the lowest pH is the strongest of the weak acids.
Your Turn
Weak acids in the ocean are lowering the ocean pH. To determine the strengths of weak acids, the hydronium ion concentration of their solutions can be measured. The H3O+ ion concentrations found in 0.010 mol L-1 solutions of acetic acid (CH3COOH), formic acid (HCOOH) and propanoic acid (CH3CH2COOH) are 4.2×10-4 mol L-1,1.3×10-3 mol L-1 and 3.6×10-4 mol L-1, respectively. Write the balanced reactions of these acids in water and determine which acid is the strongest.
The acids undergo the following reactions in water:
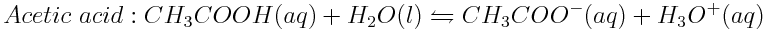
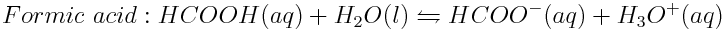
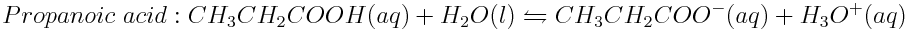
Then, determine which acid produced the greatest concentration of hydronium ions in solution and which acid produced the lowest concentration of hydronium ions. In order from strongest to weakest, the acids are arranged as follows: formic acid, acetic acid, propanoic acid.