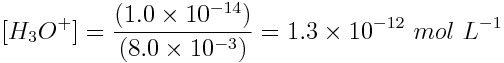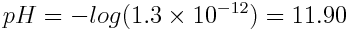Recognizing the strength of an acid or base is important in determining the pH of an acidic solution. It is relatively simple to determine the change in the pH of an aqueous solution resulting from the addition of a strong acid or base. This is because strong acids and bases are strong electrolytes, and their acid-base reaction with water goes to completion. Therefore, the initial concentration of a strong acid or base is equal to the concentration of hydronium or hydroxide ions produced upon ionization in water.
Worked Example
Hydrochloric acid is a strong acid that undergoes the following reaction with water:.
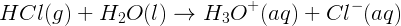
If the original concentration of HCl(aq) was 0.10 mol L-1, what is the pH of the solution?
Because HCl(aq) is a strong acid, its acid-base reaction with H2O(l) goes to completion. We know that the original concentration of HCl(aq) was 0.10 mol L-1; therefore, the concentration of H3O+(aq) that is produced is also 0.10 mol L-1. The pH of the solution can be found as follows:
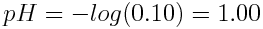
Your Turn
What is the pH of a 8.0×10-3 mol L-1 NaOH solution at 25°C? Note: NaOH is a strong base.
Because NaOH is a strong base, [OH-] = 8.0×10-3 mol L-1.

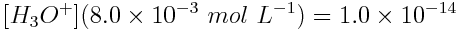
By rearranging the equation we can solve for the concentration of hydronium ion, which can be used to find the pH.