Why Should We Care?
A molecular level understanding of the behavior of acids is often used in chemistry to develop an understanding of chemical reactions. Knowledge of acids in solution allows chemists to make important predictions about the chemical reactivity of substances.
Your Turn
Understanding the molecular-level behavior of acids enables chemists to picture what happens when strong and weak acids ionize in water, including ocean water. Draw a solution containing nitric acid (HNO3, a strong acid) and a solution containing hydrofluoric acid (HF, a weak acid). Do not draw all the water molecules in solution.
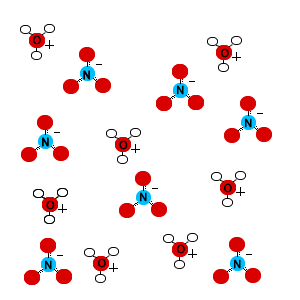
Nitric acid is a strong acid. This means it will ionize completely in solution, producing hydronium ions (H3O+) and nitrate ions (NO3-).
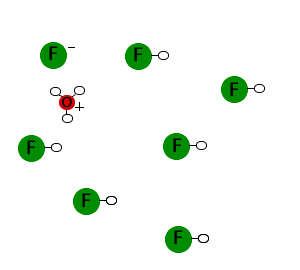
Hydrofluoric acid is a weak acid. As a result it will only partially ionize in solution. Most of the molecules remain as HF molecules, but a few ionize to form hydronium ions (H3O+) and fluoride ions (F-).