What Do We Know?
By contrast, very few of the acid molecules in solutions of weak acids are ionized. An example of a weak acid is acetic acid (CH3COOH). In solution, most acetic acid molecules remain as complete CH3COOH units. However, a few acid molecules ionize to form acetate ions (CH3COO-) and hydronium ions (H3O+). Notice that very few acetic acid molecules are ionized in the image below. Again, the many water molecules that surround each of the molecules and ions are not shown.
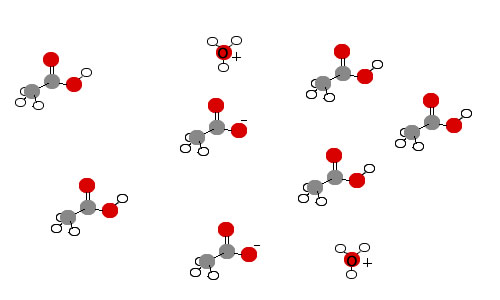
Measurements of pH indicate the concentration of hydronium ion in acidic solutions. The hydronium ion concentration can then be used to determine the extent to which an acid ionizes. The knowledge of the the extent to which an acid ionizes enables chemists to develop a molecular level picture that matches experimental evidence.