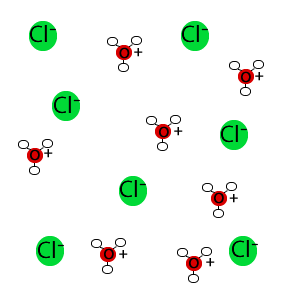
An important skill as a chemist is the ability to form molecular level pictures of chemical substances and processes. This is useful in distinguishing between strong and weak acids.
What Do We Know?
In solution, almost all the molecules of strong acids react. Based on this knowledge chemists can develop a molecular picture of strong acids. For example, because hydrochloric acid (HCl) is a strong acid, we picture HCl in solution not as units consisting of a hydrogen atom and a chlorine atom bonded together (H-Cl) but instead as separate chloride ions (Cl-) and hydronium ions (H3O+) in solution. The graphic below shows the ions that form when HCl is added to water. This is simplified by not showing the many water molecules that surround and aquate each of these ions.